We are delighted to announce that Sumitomo Riko Company Limited (Headquarters: Naka-ku, Nagoya-shi; President & CEO: Kazushi Shimizu) and Lilac pharma Inc. (Headquarters: Kita-ku, Sapporo-shi; President: Motoki Susa) have jointly developed a new “Microfluidic device (tool to manufacture lipid nanoparticles).
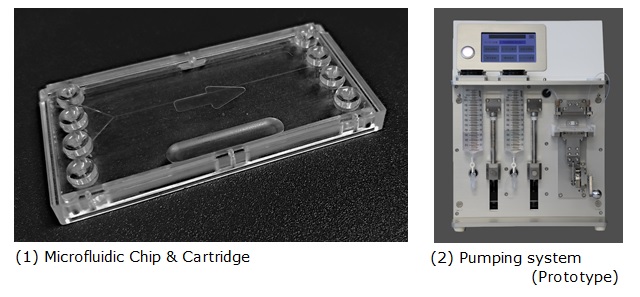
This product is composed of a set including (1) the microfluidic chip in which the lipid nanoparticles are generated and (2) the pumping system designed for precise injection of the ingredient solutions into the microfluidic chip. The microchannel fabricated in the chip is the same as that fabricated in Lilac pharma’s “iLiNP®” microfluidic chip. The iLiNP chip is designed for manufacturing high quality (homogeneous) lipid nanoparticles from a lipid solution with high reproducibility.
We will exhibit the device at the “36th Annual Meeting of the Japan Society of Drug Delivery System” to be held on August 28 and 29, as an “easy-to-operate system” for preparation of high quality lipid nanoparticles in a Lab. (Company exhibit will be held online).
* Lipid nanoparticle (liposome): a spherical vesicle with a membrane mainly composed of lipids. It can encapsulate materials such as nutrients and pharmaceutical products and protect them from degradation.
* Drug Delivery System (DDS): a technology to increase the effectiveness of pharmaceuticals and minimize side effects by controlling the distribution of pharmaceuticals in the body.
Lilac pharma Inc. was founded in 2016 as a pharmaceutical startup originating from Hokkaido University. The iLiNP microfluidic chip has original microchannel structure suitable for manufacturing lipid nanoparticles from a lipid solution. This technology is invented by professors of Hokkaido university, Professor Manabu Tokeshi, Assistant Professor Masatoshi Maeki and his colleagues. Lilac pharma has been in-licensed the iLiNP and its relating technologies since 2018.
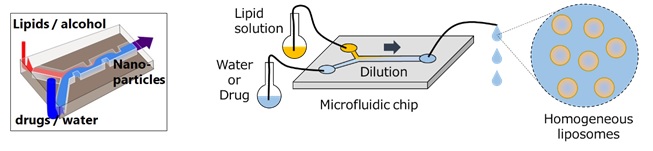
Lipid nanoparticles are made by diluting a lipid solution (raw material) with water and allowing it to self-assemble in the water. The iLiNP’s microchannel is designed to mix the lipid solution with water appropriately in the microspace, resulting in maintenance of the optimum dilution. Consequently, homogeneous (high quality) lipid nanoparticles can be produced with high reproducibility if compared with the previous batch method (stirring and diluting the solution in a tank). Furthermore, by changing the total flow rate and the flow rate ratio, the user can easily modify the dilution level and as a consequence modify the size of the particles.
http://www.lilacpharma.com/eng/proprietary_technology_eng/
Various pharmaceutical products can be encapsulated into the 10-200nm lipid nanoparticles. The encapsulation protects the pharmaceutical product in the particles from degradation in the body and the particles can be adequately delivered to any part of the body. It has been found that the encapsulation will be effective with accumulation of the particles in the affected area, maintenance of the effectiveness of pharmaceutical products and reduction of side effects.
Currently, the lipid nanoparticles are used for the development of new transformative medicines for rare diseases and vaccines. Furthermore, outside the medical field, there is increasing demand for high quality lipid nanoparticles and technology that easily reproduces and creates them. The purpose of the encapsulation of non-medical products is improving the physical property and adding the new features (dispersity, stability, permeability, etc.).
Since the 1970’s, we have developed and sold “connector seals” made of silicon rubber and other products as “sealing material” for waterproofing and attached it to the top of wire harnesses inside automobiles. Using this precision rubber molding technology, we commenced the manufacture and sale of microfluidic chips and since 2019 we started the joint development with Lilac pharma.
The microfluidic chip (1) which we have developed has been commercialized by utilizing polymer materials technology (material compounds/microfabrication) and highly transparent silicon. In comparison to the glass and resin type chips, it has become possible to supply these chips according to the requirements of the clients in a cost effective and speedy manner.
We have also developed the pumping system (2) in conjunction with the microfluidic chip to make it easier for more researchers to use the newly developed microfluidic chip. By just setting the port-equipped cartridge chip in the system, researchers can simply and quickly prepare various kinds of lipid nanoparticles without the risk of human error.
Utilizing technology developed over many years, Sumitomo Riko plans to speed up business development in the biomedical field. We will greatly strengthen our partnerships with life science research institutions and will support product development beginning with mircofluidic chips. By doing so, we aim to grow into a corporation that contributes to safety, comfort, and the environment for people, society, and the earth.
Lilac pharma Inc. was founded in 2016 as a pharmaceutical startup originating from Hokkaido University. To acquire original DDS technology, Lilac pharma has in-licensed the iLiNP microfluidic chip and its relating technology from Hokkaido university since 2018. Currently Lilac pharma collaborates with not only the pharmaceutical companies but also the companies outside the medical field to develop new nanoparticle products.
Location: Room #C, Hokkaido Collaboration Center (Collabo Hokkaido Bldg.)
North 21, West 12, Kita-ku, Sapporo-shi
On Hokkaido University campus
Capital: 14.1 million yen
Representative: President Motoki Susa
Establish: April 18, 2016
Business Content: Investigation and consultation pertaining to the research and development of pharmaceutical products and cosmetics, etc.

http://www.procomu.jp/dds2020/
Sales reservations scheduled from this winter. Price TBA.
Advanced Systems R&D Center, Sumitomo Riko Company Limited
(1, Higashi 3-chome, Komaki-shi, Aichi 485-8550, Japan)
Email: tri-health-contact@jp.sumitomoriko.com
TEL: +81-568-77-2199
Business Hours: 9:00~17:00 (except Saturday, Sunday, public holidays, Golden Week, summer holidays and end of year/new year holidays)